Preimplantation Genetic Diagnosis (PGD) and Preimplantation Genetic Screening (PGS)
![]()
Preimplantation Genetic Diagnosis (PGD) was developed in the late 1980s as an alternative to prenatal diagnosis and possible termination of pregnancy of an affected fetus for couples who are at risk of passing on serious genetic diseases to their children.
Preimplantation Genetic Diagnosis technique requires the use of the test tube baby technique (IVF) even if the couple is fertile in order to produce embryos for biopsy and testing for genetic disorders before it implants in the womb (uterus). It offers the couple at risk the chance to have an unaffected child and avoids the need for abortion. The procedure is associated with ethical and medical concerns and raises issues of sex selection and genetic engineering.
Despite an increasing number of genetic disorders that can now be diagnosed by this technique, not all-genetic disorders can be diagnosed in this way. Cystic fibrosis, monotonic dystrophy, Huntingtons disease and beta thalassaemia remain the most commonly diagnosed conditions (PGD Consortium 2012).
It should be noted that genetic disorders could be due to a single gene disorder (disorder resulting from mutation affecting individual genes on a chromosome) or abnormal number 9aneuploidy) or structure of chromosome or mitochondrial disorders (cause metabolic disorders, which are complete maternal inheritance). In single gene disorders, such as cystic fibrosis, the actual genes of the sampled embryo can be examined for the presence of the condition. Other genetic disorders, such as Duchenne's muscular dystrophy, or hemophilia, affect only males (sex linked diseases). In these cases, the cell is examined to determine the sex of the embryo and only female embryos are replaced.
In cases of recurrent chromosomal abnormalities such as Down's syndrome and recurrent miscarriages caused by parental translocations, the number and character of several chromosomes can be determined.
The world first three parent baby using a new technique that incorporate DNA from three people was born to Jordian couple treated by US team in Mexico. in 2017 Scientists in the USA convincingly demonstrated how inherited diseases caused by defective genes can be corrected in the earliest stage of life using Crispr Cas9 gene editing technology.
How is PGD performed?
Standard IVF technique is used when the procedure requires FISH analysis. ICSI is used when there is abnormal semen or PCR analysis is required. This is to reduce the risk of contamination with sperm DNA.
A single cell is removed from an 8-cell embryo (cleaved embryo) through an opening in the outer protective coat. The procedure is carried out under the microscope without damaging its ability to continue to develop normally (because at this stage of development none of the embryo cells have become specialized). The cell is then analyzed for the presence of genetic disorders. Blastocyt biopsy provides more DNA material for analysis compared with biopsy from cleaved embryo and the resulting embryos have higher implantation and live birth rate compared with cleaved embryo biopsy.
A new technique, called comparative genomic hybridisation (CGH) evaluate all 23 chromosome pairs allowing completely screened embryos to be identified and transferred (current PGD and PGS methods do not allow analysis of every chromosome and therefore a proportion of abnormal embryos can be missed). The procedure is performed on a day 5 blastocyst, biopsies are taken and analysed (blastocyst has about 100 cells). Removing cells at this stage should be less damaging, and by analysing five or six cells the clinician can be more confident that the no genetic abnormality exists in the embryo. Studies analysing the benefits of CGH to date are promising, but more scientific evidence is required before the technique is applied routinely in IVF centers
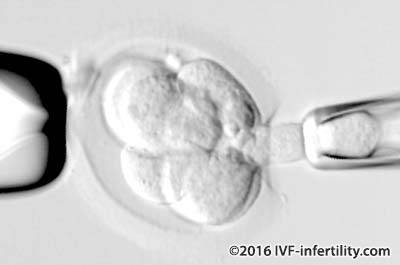
Three methods are available:
FISH (Fluorescence in situ hybridization) analysis
This technique allows specfic chromosomes to be identified by utilizing fluorescent probes which are specified for chromosomes. The labeled probes bind to chromosomes and can be visualized under a fluorescent microscope. Currently, the technique enables up to 5 chromosomes (X, Y, 13, 18 and 21) to be detected simultaneously in a single cell. This technique is used for sex determination in sex linked diseases and for numerical and structural chromosomal abnormalities e.g. aneuploidy screening.
PCR (Polymerase chain reaction)
This technique allows amplification of selected DNA fragment extracted from the nucleus and is used to detect presence or abscence of mutation of a single gene.
A diagnosis is usually obtained within a day or so of the test, and only the unaffected embryos are replaced into the uterus on day 4 or 5.
Haplotyping
This technique was developed in 2006. It uses DNA fingerprinting to identify chromosomes carrying affected genes.
To whom PGD is advised?
- Couples with repeated pregnancy loss due to genetic disorders.
- Couple who have a child with a genetic disease and are at high risk of having another.
- Couples who wish to identify a tissue match for a sick sibling who can be cured with transplanted cells such as Leukaemia
Couples with genetic disorders should receive adequate counselling before embarking on PGD. They should be informed about other options such as prenatal diagnosis and termination of pregnancy if the fetus is affected, gamete donation, remain childless and adoption. They should also be counselled about the risks of misdiagnosis and no diagnosis be made.
Unfortunately, for older women and women with high baseline FSH levels, PGD may not be a good alternative because of lower pregnancy rates. Also, they may not produce enough embryos to be tested.
Clinical experience remains limited and the test is not 100% reliable, as sometimes the analyzed cell does not represent the rest of the embryo. To date, about 1000 babies have already been born worldwide using PGD. There are no reports of increased fetal abnormalities, or low birth weight or increased perinatal death in babies born following PGD compared to babies born after IVF and ICSI . However, long-term consequences on the fetus is unknown at present.
It is important to note that there is a possibility that none of the embryos tested will be normal, in this case none would be suitable to transfer. Furthermore, as with all tests there is a possibility of a false positive and false negative result (1 in 6 and 1 in 50 respectively). This is because many embryos display inconsistency in chromosomes from cell to cell (mosaicism) and thus the cell taken for biopsy may not be a representative of the other cells of the embryo. For this reason, it is recommended that woman be tested during pregnancy (at 11-16 weeks) for chromosome abnormalities.
Few centres provide PGD services. We apologize to our viewers for not providing lists of overseas centres and we are currently looking into the matter.
The use of PGD is increasing and its indications are expanding. Though most people agree and support PGD; for some, testing for diseases that may not be fatal, or may not manifest for decades raises thorny ethical questions. The table below shows examples of genetic diseases that can be diagnosed using PGD.
A total of 712 PGD treament cycles were perdormed in the United Kingdom in 2016. 60% of women were under 35 and the live birth rates was 30% for fresh cycles compared to 36% for frozen cycle.| Autosomal Recessive, the risk to offspring 1 in 4 | Autosomal Dominant, thevrisk to offspring is 1 in 2 | Sex-linked, the risk to offspring is 1 in 2 male | Chromosomal Abnormalities |
| Cystic Fibrosis | Huntingtons Disease | Hemophilia | Translocation |
| Tay Sach's Disease | Congenital Adrenal Hyperplasia | Duchenne Muscular Dystrophy | Klienfelters Syndrome |
| Thalassemia | Marfans Syndrome | Fragile X | Aneuploidy |
| Sickle Cell Disease | Myotonic Dystrophy | X Linked Mental Retardation | |
| Spinal Muscular Dystrophy | Wiskott-Aldrich syndrome | ||
| Breast cancer (BRCA1) | |||
| Familial Adenomatous polyposis coli (FAP) | |||
| Familial Alzheimer's disease |
What is Pre-implantation genetic screening (PGS)?
Pre-implantation genetic screening (PGS), also called aneuploidy screening is very similsr to PGD but aims to improve IVF outcome, it involves screening embryos using FISH for common aneulopidy (chromosome 21, 18, 16, 13 and X and Y).
Aneuploidy is a condition where the embryo contains the wrong number of chromosomes in each cell, either a loss or a gain of a chromosome.The common aneulopidies account for 90% of genetic miscarriage and birth defects. The doctor then selects only the chromosomally normal embryos for embryo transfer. In the UK the procedure is licensed for use in older women where the incidence of chromosomal abnormalities such as Down's syndrome increases by seven to seventy fold, women with a history of recurrent miscarriages, or repeated IVF failure (with good quality embryos) in order to increase the IVF live birth rates. Evidence is lacking that PGS improves the live birth rates in older women and two recent randomised trials found no improvement in live birth rates in older women and it may lower pregnancy rates.
Bhatt and colleagues from USA reported that the use of PGS-A was associated with improved live birth rates in couples with recurrent pregnancy loss undergoing frozen embryo transfer (human reproduction 2021)
Outcome of PGD and PGS
The outcome of PGD and PGS depends on:
- The number of embryos available for biopsy which decline as the woman gets older and in woman who had poor ovarian reserve
- The genetic condition, because the proportion of embryos likely to be not affected (normal embryos) varies with the genetic disease.
How safe is PGD and PGS?
PGD and PGS have been associated with a significant risk of perinatal death compared with ICSI (4.6% compared to1.9% respectively). However, recent data from Brussels, Belgium on 995 babies born through the technique between 1993 and 2008 suggested the risks of low birth weight, premature birth, major malformations and the perinatal death rate was the same as for other forms of IVF (ESHRE meeting 2012).
| X-linked disease or gender selection | Chromosomal abnormalities | Pre-implantation genetic screening (PGS) | |
| Treatment cycles | 638 | 1244 | 3257 |
| Biopsied embryos | 3885 | 8218 | 17899 |
| Embryos transferred (ET) | 463 (73%) | 89% | 85% |
| Clinical pregnancy | 114 | 175 | 522 |
| Clinical pregnancy per cycle | 18% | 14% | 17% |
| Clinical pregnancy per ET | 25% | 21% | 25% |
References
ESHRE consortium 2005
Brudie and Flinter BMJ 2007
Mustenbrook et al New England Journal of Medicine 2007
UK centres that are licensed for PGD
University College Hospital London
Wolfson family Clinic at Hammersmith Hospital, London
Clarendon Wing, Reproductive Medicine Leeds
Fertility Centre at life, Newcastle
The Assisted Conception Unit , Glasgow Royal Infirmary
UK centres that are licensed for PGS
The Assisted Reproduction and Gynaecology Centre
Reproductive Genetics Institute