Ovarian Reserve
Ovarian reserve is a term used to describe the number of eggs remaining in a woman's ovaries at a given time. As a woman ages, her supply of eggs gradually declines over time until the eggs are depleted at menopause. Some women have an ample supply of eggs while others may have only a few. The number of eggs in the ovaries is set at birth and the rate of continuous depletion of the eggs throughout life, is determined by genetic factor.
Ovarian reserve is an assessment of how likely the woman is to get pregnant. Testing for ovarian reserve is helpful for providing adequate counselling of IVF treatment outcome, and very helpful in tailoring ovarian stimulation protocols.
It is well known that pregnancy rates in IVF are directly dependent upon the quality and number of embryos transferred, and the more eggs a woman grows, the better the embryos to select from and replace This is why women with a good ovarian response have higher pregnancy rates than women with a poor ovarian response. Impaired ovarian reserve was found not to contribute significantly to decreased fertility among young women (Hvidman 2014).
What is reduced (poor) ovarian reserve (ROR)?
There is no agreed definition. However, ROR is usually defined by the presence of at least two of the three following features (Bologna criteria).
- Advanced maternal age (over 40 years old)
- A previous poor response to ovarian stimulation (three or less eggs).
- An abnormal test of ovarian reserve.
What is the incidence of poor ovarian reserve?
Approximately 10-20% of women undergoing IVF have poor ovarian reserve and about 10-18% of IVF cycles result in poor response.
What are the causes of reduced ovarian reserve?
- Natural decline of ovarian reserve due to advanced maternal age
- Genetic factors, such as Fagile x syndrome
- Autoimmune disorders
- Following radiotherapy and chemotherapy
- Previous ovarian surgery such as thermal ablation of endometriosis and laparoscopic ovarian drilling
- Enviromental factors such as smoking.
- High body mass index.
- Unexplained (possible causes include: FSH antibodies and decrease FSH receptors on granulosa cells
How is ovarian reserve measured?
Testing for ovarian reserve is not the same as testing for ovulation.To date, there is no reliable test available. However, current methods of assessment include:
- Day 2-4 FSH, LH and estradiol: these are the most commonly used tests to assess ovarian reserve. Persistent high levels of FSH suggest reduced ovarian reserve. However, there is inter-cycle variation and the levels vary depending on cycle day. Although these tests have a moderate predictive value of ovarian response they are not predictive of pregnancy. High FSH strongly predicts poor IVF response in older women, less so in younger women. There is no absolute values that define how high an FSH level can be and still achieve pregnancy due to variations in laboratory assessments and treatment methods. Furthermore, a normal day-three FSH level cannot guarantee adequate response to ovarian stimulation. High estradiol levels are associated with poor response and lower pregnancy rates. However, it also has a low predictive value(Domingues et al 2010).
- Antimullerian hormone (AMH).This hormone is produced by the ovaries (from the granulosa cells of pre-antral follicles) and hence serum AMH levels correlate with follicle pool. AMH levels decline sharply with advancing maternal age, they are not influenced by cycle day so can be performed any day of the cycle. The result is not affected by previous hormone treatment such as contraceptive pills. Furthermore, there are minor inter-cycle variabilities compared with FSH, LH and Estradiol. AMH levels appear to be the best hormonal test for ovarian reserve. AMH levels can predict poor and over responders and thus allows optimal stimulation protocol for IVF treatment. A level of less than or equal to 5.0 pmol/l usually indicate a low response while a level greater than or equal to 25.0 pmol/l will indicate high response. A recent study found women with high AMH levels were 2.5 times more likely to have a successful IVF cycle than women of a similar age with low levels of the hormone. The researcher commented that AMH levels were a predictor of pregnancy and live birth, even when the mother's age and egg production were taken into account (JCEM, 2013). It appears that although AMH level is a good predictor of ovarian response, AMH levels are not good predictor for pregnancy outcomes in IVF in young women. Furthermore, pregnancies were reported in women with undetectable AMH levels. Currently, AMH is the only widely accepted test to predict ovarian reserve before and after chemotherapy.
- Inhibin B: Inhibin-B is an ovarian hormone, and is produced by small antral follicles in response to FSH stimulation. Inhibin B levels decline with advancing maternal age due to both fewer follicles and decreased secretion by the granulosa cells.
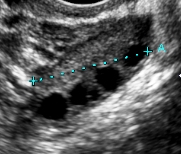
- Antral follicle count(AFC). This measures the number of follicles between 2-10 mm in diameter in each ovary. Vaginal ultrasound is the best way to accurately assess AFC. The test is easy-to-perform, quick and cheap. AFC between 8-12 indicates normal reserve while AFC of 4 or less indicates poor reserve.Recent studies have not shown evidence of benefit over AMH measurement.
- Ovarian volume: the ovary reduces in size with increasing age, regardless of whether the woman has given birth. Assessment of ovarian volume by ultrasound scan appears to only hold in women aged 35 years or older.Ovarian volume at a cut of value of 3 ml predictive of poor response. measuring ovarian volume is not recommended by NICE in the UK .
- Provocative tests: The rationale of these tests is to challenge the ovaries with clomiphene or GnRH agonist and assess whether they have responded appropriately by measuring the hormone levels before and after. However, the predictive value of these tests is no better than FSH or AMH.
Treatment options for women with reduced ovarian reserve
There is no individual treatment protocol that appears to offer any clear advantages in POR. There is a need for well designed prospective randomized controlled studies, which are adequately powered to find out the best protocol. Patients should receive individualized treatment based on their ovarian reserve status and any other factors involved in their infertility condition such as male factor and autoimmune abnormalities
- Natural cycle IVF, this have the advantage of being cheap, a pregnancy rates of around 10% is to be expected. The major drawback is the risk of premature LH surge and spontaneous ovulation in 30-50% of cycles.
- IVF with mild ovarian stimulation protocol
- IVF with short micro dose agonist protocol and high dose gonadorophins
- IVF with antagonist protocol
- IVF with double stimulation and egg collection, this protocol has been advocated by by Professor Kuang and his team at the Shanghai Ninth People's Hospital affiliated to Shanghai Jiao Tong University School of Medicine, China (2013).
- Some clinicians recommend adding one or more of the following drugs to ovarian stimulation regimens:
- Aspirin: The rationale is to improve ovarian blood flow. Aspirin is not advisable to take if patient suffers from asthma, stomach ulcers or known blood disorders
- Steroids, these are also associated with risks such as difficulty sleeping at night. Peptic ulcer, osteoporosis etc.
- Growth hormone.
- Estrogen, pretreatment with estradiol or combined oral contraceptive pills for 3 weeks prior to ovarian stimulation with gonadotropins.
- Androgen supplementation (DHEA): A recent clinical trial by the Center for Human Reproduction in New York showed significant effectiveness. after two to six months of treatment of 25 mg daily. Gleicher and Barad(2006) reported better response in women with poor ovarian reserve taking 25 mg three times daily for 18 weeks. Jindal and Singh (2014) reported improvement in ovarian response in known poor responders who had previous failed IVF and ICSI cycles by taking DHEAS 75 mg orally daily for up to 6 months prior to IVF. More recently Kotb et al 2016 reported higher clinical pregnancy rates in women with poor ovarian reserve who took DHEA for three months before their IVF. The rational for DHEA is that primordial follicles needs androgen for their growth and it appears that women with reduced androgen have reduced ovarian reserve In summary, DHEA 25mg three times a day for 8 weeks prior to IVF cycle. the reason for giving the medication for 8 weeks or longer is that primordial follicles takes 2-3 months to become mature follicles. It is also advised that patient who takes DHEA should not take contraceptive pills as it reduce androgen production, and should be in short protocol treatment with minimal suppresion agonist or antagonist during IVF cycle.
- Metformin 1000-1500 mg/day start 1-3 months prior to initiating IVF treatment.
- Stem cell technology and mitochondrial transfer may offer help in treatment of ROR in the future
- IVF with donor eggs offers a reliastic treatment option when the ovaries fail. Not only IVF with donor eggs from a younger, fertile donor results in higher pregnancy rates approaching 60-70% but also reduction in the risk of miscarriage and chromosomal abnormalities such as Down syndrome. Obviously,donor eggs will not be acceptable to everyone with poor ovarian reserve but the alternatives are childfree living and adoption.